FDA approves Pfizer and Moderna booster doses, authorizes mix and match for COVID-19 vaccines
Author

Blaire Bryant
Upcoming Events
Related News

Key Takeaways
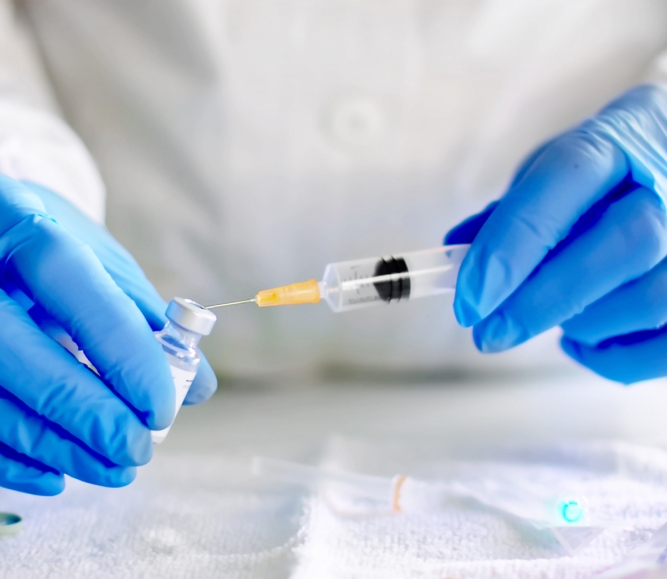
On October 21, the Centers for Disease Control and Prevention (CDC) endorsed the use of Moderna and Johnson & Johnson booster shots following a recommendation from the Advisory Committee on Immunization Practices’ (ACIP). The endorsement follows the Food and Drug Administration’s (FDA) October 20 announcement approving the emergency use of booster doses for both vaccines.
The CDC also endorsed the use of heterologous (mix and match) COVID-19 vaccine booster shots, following FDA approval based on safety and efficacy data. Eligible individuals will be able to select their booster dose from any of the FDA endorsed or approved COVID-19 vaccines.
Updated guidelines for the use of a COVID-19 vaccine booster dose are as follows:
- Johnson & Johnson COVID-19 vaccine: A single booster dose may be administered at least two months after completion of the single-dose primary regimen to individuals 18 years of age and older.
- Moderna COVID-19 vaccine: A single booster dose may be administered at least six months after completion of the primary series to individuals that are 65 years of age and older, 18 through 64 years of age at high risk of severe COVID-19, or 18 through 64 years of age with frequent institutional or occupational exposure to COVID-19.
- Pfizer-BioNTech COVID-19 vaccine: A single booster dose may be administered at least six months after completion of the primary series to individuals 18 through 64 years of age with frequent institutional or occupational exposure to SARS-CoV-2.
As key providers of local public health services and frontline service providers for the medically vulnerable, counties have supported over 190 million vaccinations in the U.S. to date and will continue to play an essential role in the administration of COVID-19 vaccines and boosters. For more information on how county health facilities can prepare for the distribution of COVID-19 boosters, see the “Vaccine Booster Readiness Checklist for County Health Facilities” tab in NACo’s COVID-19 Vaccine Distribution Toolkit.
ADDITIONAL RESOURCES
Resource
COVID-19 Vaccine Toolkit for Counties
Related News

HRSA offers funds to aid care transitions for justice-involved individuals
On April 10, the U.S. Department of Health and Human Services’ Health Resources and Services Administration (HRSA) announced the availability of $51 million in funding opportunities open to HRSA-funded health centers. HRSA-funded health centers, which serve over 30 million patients, play a crucial role in county healthcare systems emphasizing equity and accessibility in healthcare. This new initiative focuses on supporting individuals leaving incarceration by providing health services during the critical 90 days before release, assisting justice-impacted individuals with their return to the community by expanding access to primary healthcare—including mental health and substance use disorder treatment.

Join NACo in celebrating County Health Day on April 19, 2024
County Health Day, which falls during National County Government Month, celebrates the pivotal role counties play in promoting public health and building resilient communities.

FCC takes critical steps to improve the 988 National Suicide Lifeline
On March 21, bipartisan congressional leaders and FCC Chairwoman Jessica Rosenworcel announced steps to improve the 988 National Suicide Lifeline. This announcement marks major progress on the nation’s crisis response, a priority for counties and a key policy pillar of the NACo Commission on Mental Health and Wellbeing.